From FDA Clearance to
CE Marking: Bridging the Gap
qtec Healthcare Solutions – Your Fast Track to the European MedTech Market
You’ve cleared the FDA – now what? For many U.S. manufacturers, expanding into the European market comes with unexpected regulatory hurdles. While a 510(k) or PMA may open doors in the U.S., it’s rarely enough to meet the detailed and evolving requirements of the EU MDR or IVDR.
That’s where we come in. At qtec, we specialize in translating your U.S. documentation into a strategic, streamlined pathway to CE certification. We identify regulatory gaps, close them efficiently, and get your products market-ready – quickly, compliantly, and with confidence.

Typical Gaps Between U.S. and EU Requirements
Even highly experienced U.S. MedTech teams are often surprised by the depth and structure required under the MDR. Here are just a few of the common gaps we see:
- Technical Documentation:
The MDR demands a formalized structure, including detailed risk management, usability evidence, and clinical evaluation – far beyond most FDA submissions. - Design Control Documentation:
The EU requires a design and development plan aligned with MDR Annex II – often missing or fragmented in U.S. DHFs. - Post-Market Surveillance & Vigilance:
The EU mandates robust PMS, PMCF, and trend reporting activities that go beyond FDA expectations. - Clinical Evaluation:
Clinical evidence must follow a defined methodology, not just literature or bench data. - Labeling & Language Requirements:
Instructions for use and labeling must be translated into all EU market languages – and meet format expectations. - Quality System Documentation:
Even with ISO 13485 certification, documentation depth, SOP alignment, and audit readiness are often not at MDR level.
What We Offer – Full-Scope Regulatory Support for U.S. Companies
qtec helps you turn your FDA clearance into a strong foundation for your CE strategy. Whether you’re planning your first European launch or scaling up existing EU operations, we deliver end-to-end support tailored to your documentation, timelines, and business goals.
Regulatory Affairs
- Gap analysis: FDA vs. MDR/IVDR
- Technical file creation and remediation
- Direct liaison with Notified Bodies
- EU regulatory strategy based on your existing U.S. data
Quality Management Systems
- Implement or adapt their QMS to be MDR-compliant
- Audit preparation and support
- CAPA systems, supplier controls, internal audits
Design Control
- EU-aligned design documentation and DHFs
- Verification & validation documentation support
- Project planning in line with MDR expectations
Language & EU Market Specifics
- EU labeling, IFU translations, UDI, and EUDAMED registration.
- Coordinate translations, EU rep services
- Assist with UDI and PRRC (Person Responsible for Regulatory Compliance)
Clinical Affairs
- Clinical Evaluations (CEP & CER)
- Post-Market Surveillance & Vigilance
- PMCF strategies, templates, and implementation
- EU-compliant clinical study planning

Why qtec? Because Expertise and Speed Matter
We speak both regulatory languages – FDA and MDR. Our team has supported countless U.S. manufacturers in entering the EU market smoothly, efficiently, and with the right long-term strategy. We understand how to optimize what you already have and fill in the gaps – with minimal disruption and maximum momentum.
Whether you’re a start-up or global brand, we bring structure, clarity, and speed to your EU entry.
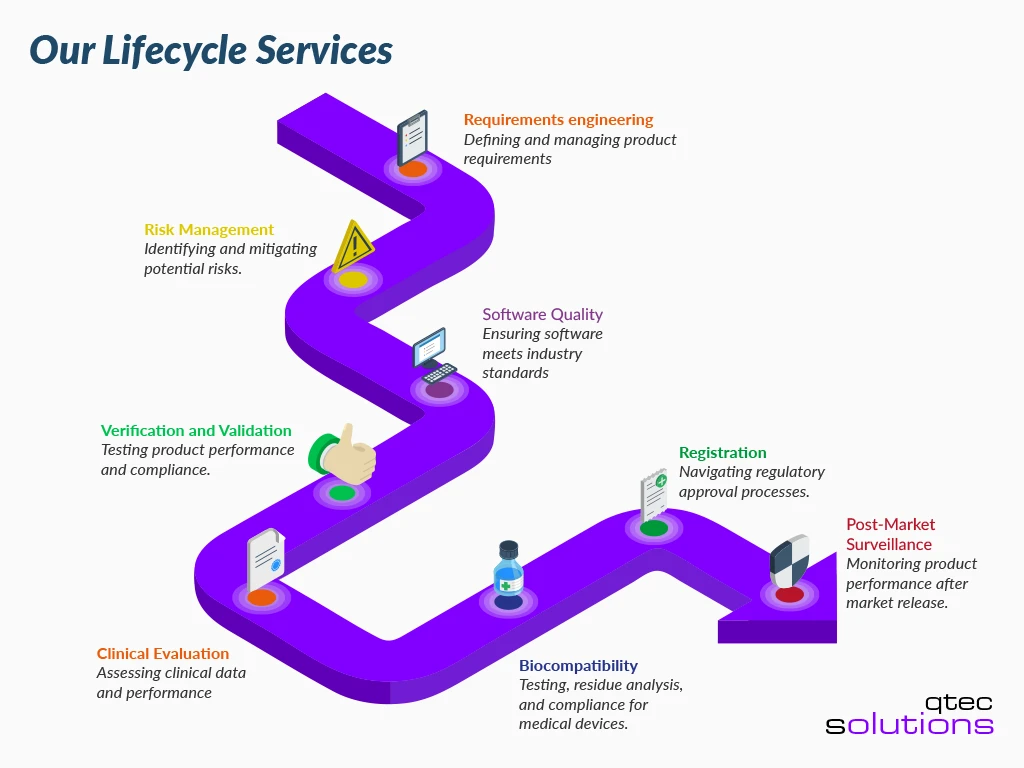
Our Lifecycle Services – Every Step Covered
From initial market strategy to post-market compliance, qtec provides comprehensive lifecycle support for your medical devices and IVDs.
We don’t just guide you through CE marking, we stay with you to ensure lasting compliance and market success.
Sign up for our newsletter now
To receive our newsletter, please register using the form. Your data is in safe hands with us and will be kept completely secure.
By clicking on the “Sign up” button, you confirm that you wish to subscribe to our newsletter and consent to the processing of your data for this purpose. In particular, you also consent to the analysis of the newsletter using so-called anonymized tracking pixels. You can revoke your consent at any time with future effect. Detailed information about the newsletter can be found in our privacy policy.

The qtec group – Your Partner for Regulatory Excellence
qtec Healthcare Solutions is part of a strong alliance: the qtec Group brings together four specialized units that collectively cover all aspects of product approval and quality assurance in medical technology. Benefit from our combined expertise and many years of experience.

Our expert knowledge for your worldwide market approval
We have in-depth expertise in the approval of Medical Devices – worldwide. From Europe to the Middle East and Africa to China and the Americas: Our experts know the respective country-specific requirements and regulations and are eager to support you with their know-how.